Biopharmaceuticals, or biologics, are pharmaceutical drug products that are
made in, taken from, or semisynthesised from biological sources. They
come in a wide range of forms, from vaccines to gene therapies. They are
used to treat several pathologies, such as cancer (Zhou et al. 2014),
autoimmune diseases (review by Rosman et al. 2013), and chronic
inflammatory diseases (review by Gils et al. 2017). Although these new
biologic therapies have now been prescribed to many patients, there
remains a lack of routine clinical assessment of the drugs levels and
immunogenicity in the serum. Below are a list of biopharmaceuticals with
links to relevant assessment tests.
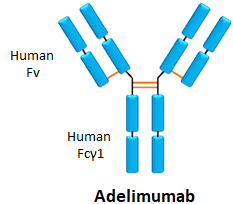
- Adalimumab is the most used of the biologics for the treatment of
- It is an anti-TNF antibody that counteracts inflammation.
- It is administered through subcutaneous injection every 2 weeks. -In 2016, its sales in Europe amounted to €2.8 billion. - A high percentage of patients form ADAs against this drug, between 20-53%.
- Labs that test for drug level- Karolinska University Lab, University of Tours, KU Leuven, Sanquin,
- Labs that test for ADA titre- Karolinska Institutet, Karolinska University Lab, KU Leuven, Sanquin, Sheba Medical Centre
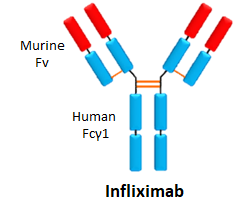
- Infliximab is a treatment option for treating
- It is administered through intravenous infusion every 6-8 weeks.
- It is an anti-TNF immunosuppressive that binds and clears TNF European sales in 2016 were €1.5 billion
- It has an immunogenicity rate of 60%
- Labs that test for drug level- Karolinska University Lab, University of Tours, KU Leuven, Sanquin
- Labs that test for ADA titre- KU Leuven, Sanquin, Sheba Medical Centre
- Labs that test for immune complexes: Karolinska Institutet
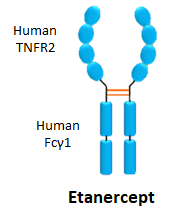
- Etanercept is used to treat
- It is an anti-TNF biologic that differs from adalimumab and Infliximab, as it contains a TNFR2 structure to bind TNF instead of a classical Fab antibody segment
- It is administered through subcutaneous injection once a week.
- In 2016, €1.6 billion was spent on this drug in Europe.
- It has an immunogenicity rate of 3-6%.
- Labs that test for drug level- Karolinkska Univeristy Lab, University of Tours, KU Leuven
- Labs that test for ADA titre- Karolinska Institutet, KU Leuven
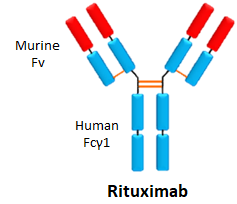
- Rituximab is used for many autoimmune diseases, such as rheumatoid arthritis and multiple sclerosis, as well as cancer.
- It binds CD20 on B cells, which results in recruitment of other immune cells and destruction of the B cells.
- It is administered through intravenous infusion every 6 months.
- Sales in 2016 were €1.4 billion in Europe.
- Immunogenicity rates varies depending on disease, 10%-30%.
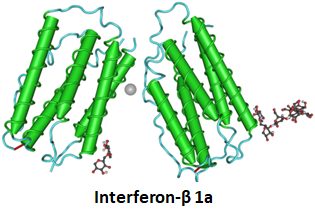
- Interferon-β is a first-line therapy for the treatment of multiple sclerosis.
- It balances the expression of pro- and anti-inflammatory agents in the brain, and reduces the number of inflammatory cells that cross the blood–brain barrier.
- It is administered through intramuscular or subcutaneous injection once per week.
- In Europe, sales in 2016 amounted to €4.3 billion.
- It has an immunogenicity rate of 28% to 47% for IFNbeta-1b-Betaseron and Extavia, 12% to 28 % to IFNbeta-1a-Rebif, and 2% to 6% to IFNbeta-1a-Avonex
- Labs that test for drug level- Bulgarian Academy of Science, San Luigi Gonzaga University Hospital
- Labs that test for ADA titre- Karolinska Institutet, Innsbruck Medical University, San Luigi Gonzaga University Hospital, Bulgarian Academy of Science
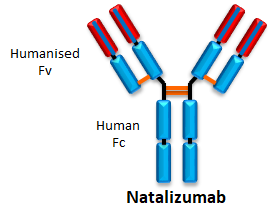
- Natalizumab is a second-line treatment for multiple sclerosis.
- It acts by binding to α4β1-integrin and blocks its interaction with VCAM-1. This results in inhibition of leukocyte migration into the central nervous system.
- It is administered through intravenous infusion every month.
- In 2016, it sold €347 million in Europe.
- It has an immunogenicity rate of 6-9%.
- Labs that test for drug level- Innsbruck Medical University
- Labs that test for ADA titre- Karolinska Institutet, Innsbruck Medical University, San Luigi Gonzaga University Hospital, IBIMA
Copyright © All Rights Reserved